It is a proud moment that a total of 18 research achievements from Chinese scholars were selected for this congress. Among them, a randomized clinical study on the EndoFresh® Single-Use Electronic Colonoscope, led by Dr. Tian Yuan's team from Peking University First Hospital, was successfully selected for the poster presentation session. It stood out from 1,175 global abstracts to become one of the 15 Chinese poster presentations!
Study Title:
"A Randomized Controlled Trial Assessing the Safety, Efficacy, and Patient Comfort of a Single-Use Electronic Colonoscope in Clinical Diagnosis and Treatment"
Study Highlights:
-
The world's first published large-scale Randomized Controlled Trial (RCT) on a single-use electronic colonoscope.
-
✅ Confirmed non-inferiority to traditional colonoscopes in terms of Cecal Intubation Rate and Adenoma Detection Rate (ADR).
-
✅ Completely eliminates cross-contamination, enhancing clinical operational safety.
-
✅ Demonstrated excellent patient pain scores and better overall comfort.
Core Data Overview:
-
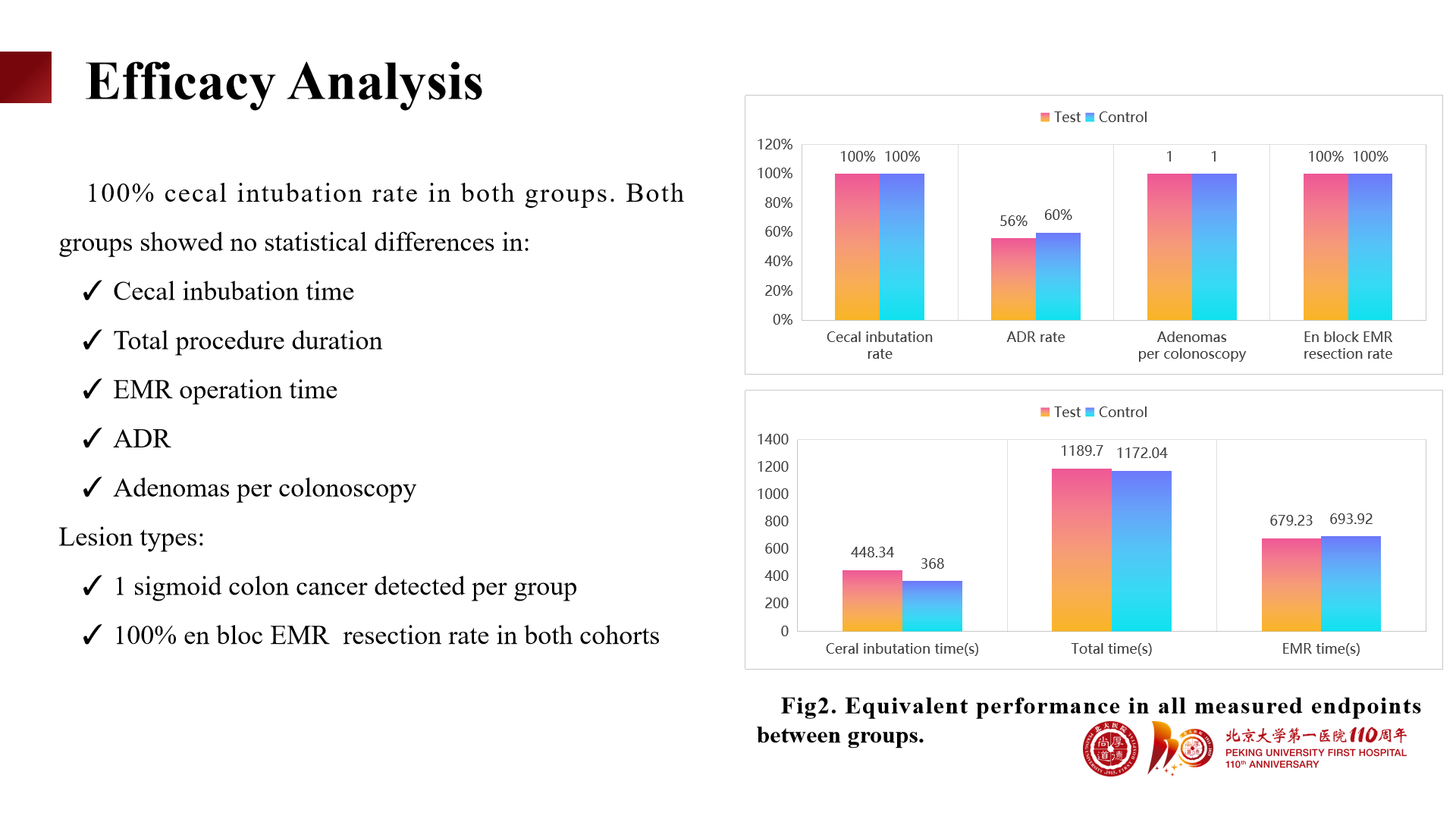
Cecal intubation rate reached 100% in both groups.
-
No statistically significant differences were found between the two groups in cecal intubation time, total procedure duration, EMR operation time, ADR, and adenomas per colonoscopy.
-
One case of sigmoid colon cancer was detected in each group.
-
En bloc EMR resection rate reached 100%.
-
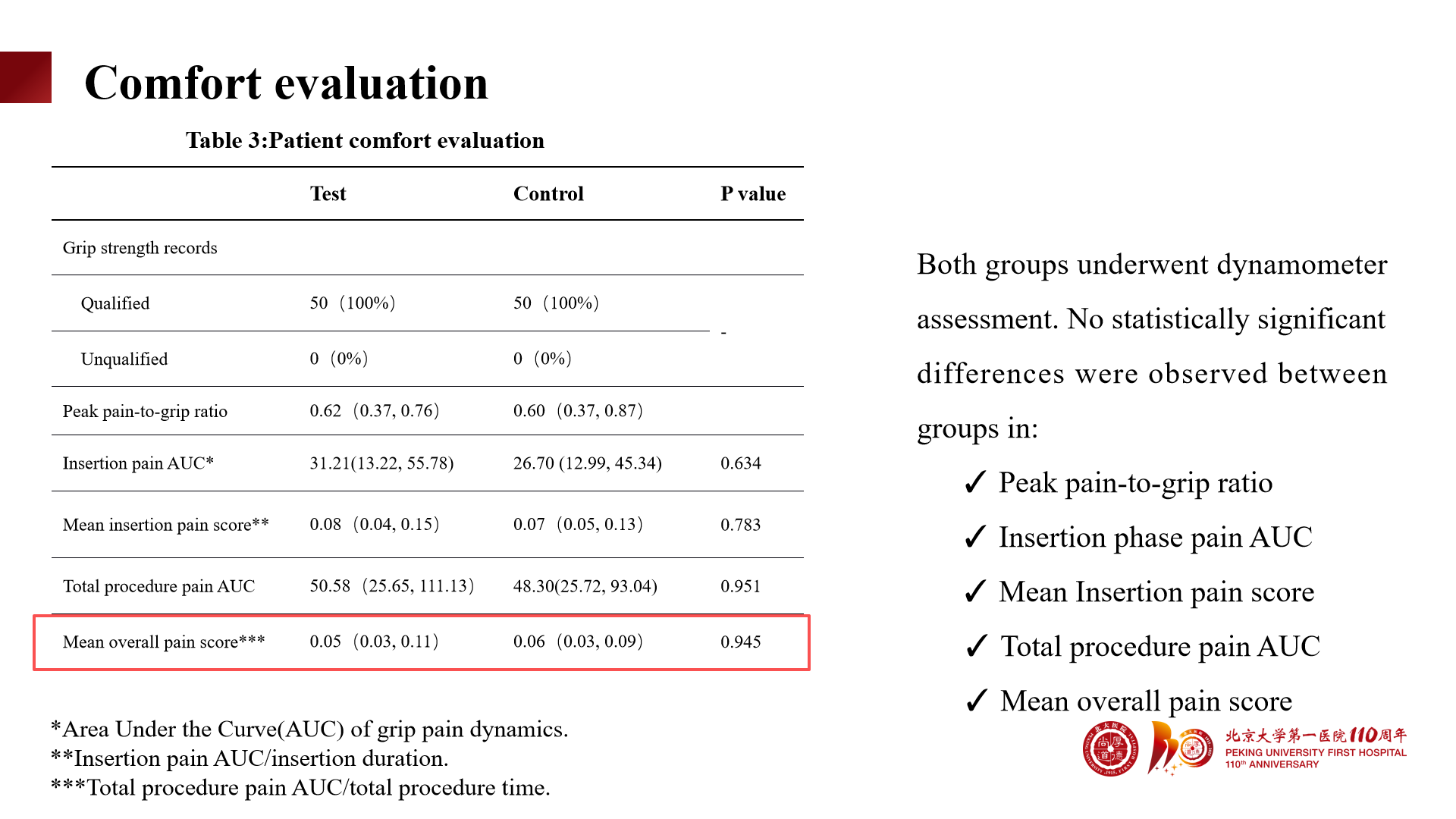
No significant difference in patient pain AUC scores; the average overall pain score was favorable (0.05 vs 0.06).
Device Highlights:
The EndoFresh® Portable Image Processor GEC-2000P compared to traditional equipment:
-
62% reduction in volume
-
54% reduction in weight
This device possesses all the functions required for routine colonoscopy and biopsy. It is particularly suitable for medical institutions and departments that currently lack endoscopy capabilities but need configuration (e.g., Emergency Departments, Operating Rooms), including institutions hoping to control endoscope procurement and maintenance costs (e.g., private clinics).
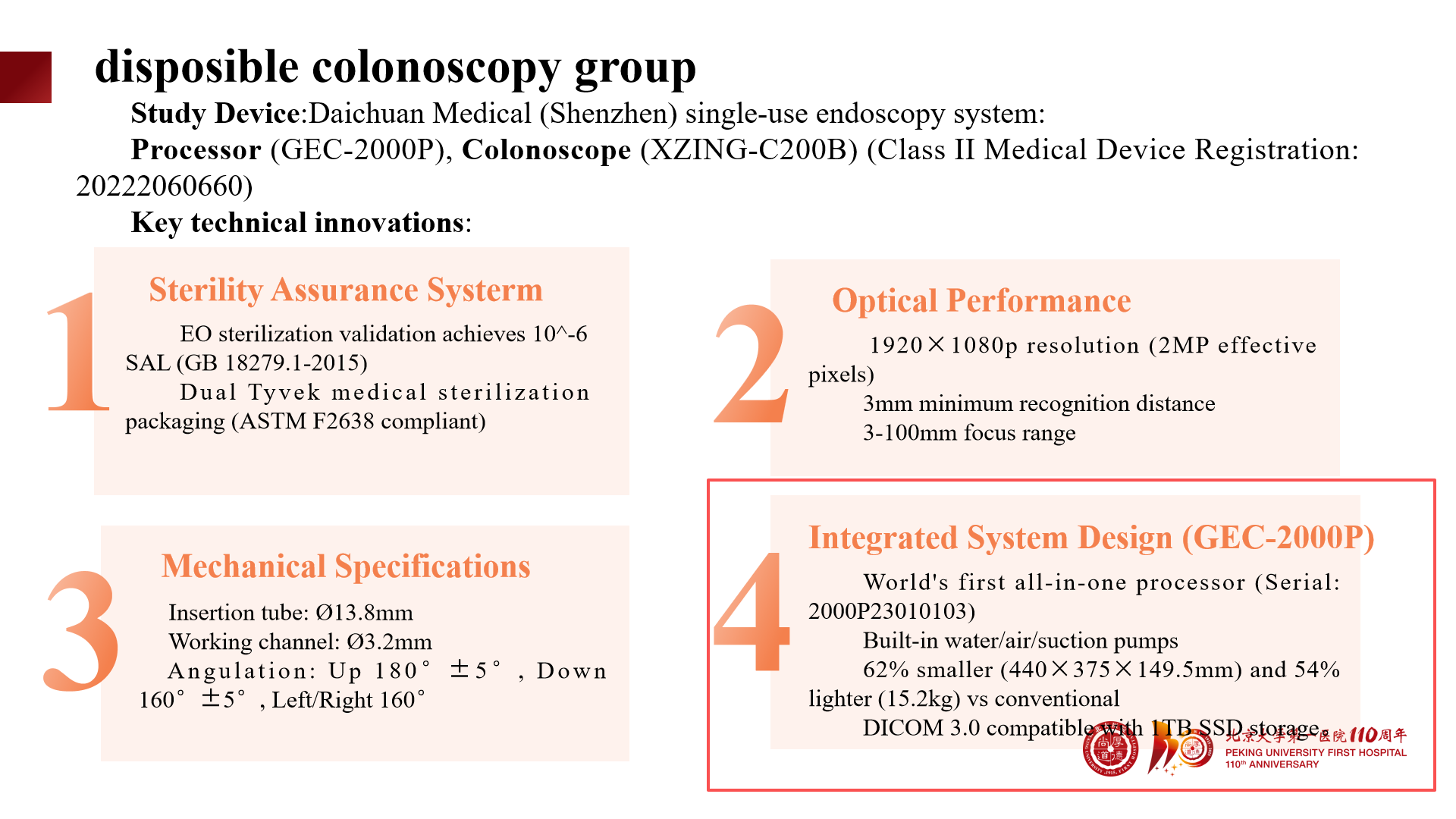
The single-use colonoscope model XZING-C200B used in this study has obtained China NMPA Class II Medical Device Registration Certificate (Registration Certificate No.: 20222060660), possesses fully independent intellectual property rights, and also holds CE and FDA certifications.
Key Technological Breakthroughs:
-
The sterile assurance system has passed EU verification, achieving a 10⁻⁶ Sterility Assurance Level (SAL) (GB 18279.1-2015).
-
Dual medical-grade sterile packaging, compliant with ASTM F2638 standard.
The Voice of China on the International Stage:
WCOG 2025 was held in conjunction with the Australian Gastrointestinal Disease Week this year, featuring speeches from over 20 internationally top experts and continuing education courses. The presentation of the EndoFresh® single-use electronic colonoscope randomized controlled study not only represents the innovative strength of Chinese enterprises in the digestive endoscopy field and validates the product's efficacy and safety through standardized clinical research but also marks a significant milestone for "Made intelligently in China" ascending the international high-end medical stage.
Clinical Significance and Future Outlook:
Following the gradual acceptance of single-use gastroscopes in the upper GI treatment field, the emergence of the single-use electronic colonoscope, which demands even higher clinical manipulation skills, will significantly mitigate the hidden risk of cross-contamination in colonoscopy diagnosis and treatment. It is particularly suitable for clinical surgeries with high infection control requirements, combined laparoscopic-endoscopic surgery, intestinal microbiota transplantation, and immunocompromised or multidrug-resistant patient populations. It enhances patient experience and medical safety without compromising operational efficiency.
In the future, EndoFresh® will continue to adhere to the philosophy of "Innovation Leadership, Patient-Centricity", collaborating with global experts to promote the inclusive development of digestive endoscopy technology, contributing Chinese wisdom and solutions towards building a safer, more efficient, and more humane global healthcare system.
